Calcium-Triggered Arrhythmias
Héctor H. Valdivia, MD, PhD, is an established investigator leader in the field of excitation-contraction coupling of the heart and calcium release channels/ryanodine receptors.
Since its inception in 1994, his laboratory has been continuously funded by the National Institutes of Health to investigate the normal and pathogenic role of ryanodine receptors in cardiac physiology and disease.
His research has led to the discovery of a novel family of ryanodine receptor ligands with potential therapeutic value to treat cardiac arrhythmias.

Calcium Signaling in the Normal and Diseased Heart
Dr. Valdivia’s lab uses transgenic animal models of catecholaminergic polymorphic ventricular tachycardia (CPVT) and other arrhythmogenic cardiomyopathies to understand the molecular and cellular mechanisms underlying congenital calcium-triggered cardiac arrhythmias and to develop novel therapies.
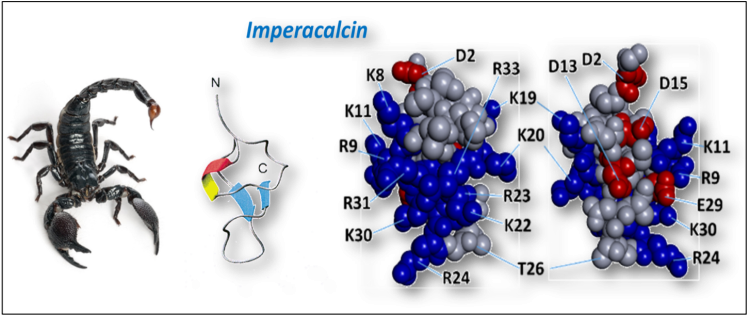
Dr. Valdivia discovered imperacalcin, a scorpion peptide and founding member of the calcin family of ryanodine receptor ligands. He is using these peptides as lead molecules to develop pharmacological therapies to treat disturbances of calcium homeostasis in the heart, including CPVT and other arrhythmogenic syndromes.
Research Team

We are looking for motivated fellows, preferably at the post-doctoral level, to join our lab for a NIH-funded research experience in cardiovascular physiology and pathophysiology. If you are interested, please email Dr. Valdivia.
Active Projects
- Partial Depletion of SR Calcium by RyR Agonists Prevents Calcium-Dependent Arrhythmias
Hyperactivity of the cardiac ryanodine receptor/calcium release channel of the sarcoplasmic reticulum (RyR2) may lead to ventricular fibrillation and other lethal cardiac arrhythmias, such as occurs in the arrhythmogenic syndrome catecholaminergic polymorphyc ventricular tachycardia (CPVT), a congenital disease affecting children and young adults with an apparently normal heart. β-blockers are the standard therapy for CPVT, but they often result in incomplete protection.
We discovered that selected scorpion venoms contain calcins, a family of peptide ligands of RyR2 that are able to penetrate cellular membranes and mitigate calcium-dependent arrhythmias due to RyR2 hyperactivity.
In this study, we have developed a rationalized and novel paradigm for the treatment of CPVT based on calcins. These results may be applied to other cardiomyopathies where controlled unloading of intracellular calcium may be desirable.
- Rational Design from Cryo-EM Structures of High-Affinity Ryanodine Receptor Ligands from Natural Peptides
Ryanodine receptors (RyRs) are sarcoplasmic reticulum Ca²⁺-release channels essential for excitation–contraction coupling in muscle. Mutations in the cardiac isoform, RYR2, cause catecholaminergic polymorphic ventricular tachycardia (CPVT), an arrhythmogenic disorder driven by aberrant intracellular Ca²⁺ handling. CPVT exemplifies how RyR2 dysfunction promotes diastolic SR Ca²⁺ “leak” and spontaneous Ca²⁺ release (SCR), key triggers of ventricular arrhythmias in a wide range of cardiomyopathies.
Calcins are a family of globular peptides that bind RyRs with exceptional affinity and specificity. Imperacalcin (IpCa), the prototypical calcin, accesses the RyR2 cytosolic vestibule and binds near the transmembrane pore, inducing a prolonged subconductance state. In CPVT models, IpCa enters myocytes, partially reduces SR Ca²⁺ load, suppresses SCR, and prevents adrenergically driven arrhythmias. Guided by structural insights, engineered IpCa analogs may function as the first high-affinity RyR blockers capable of mitigating pathological SR Ca²⁺ leak.
Aim 1 will use cryo-EM structures of calcin-RyR complexes and complementary functional assays to identify the structural determinants that confer high-fidelity RyR binding and to engineer calcin analogs capable of blocking RyR ion conduction. Aim 2 will use CPVT mouse lines prone to SCR under sympathetic stimulation and a new rabbit CPVT model with constitutive SR Ca²⁺ leak and adverse remodeling to test whether native or engineered calcins can prevent RyR2-dependent arrhythmias in vivo.
This integrative program seeks to establish a new therapeutic strategy for Ca²⁺-dependent arrhythmias, an area with limited pharmacologic options.
Funding Support
Dr. Valdivia's research is supported by these National Institutes of Health grants:
- Partial Depletion of SR Calcium by RyR Agonists Prevents Calcium-dependent Arrhythmias. R01-HL167195-01 (Valdivia HH, Alvarado FJ, PIs)
- Rational Design from Cryo-EM Structures of High-Affinity Ryanodine Receptor Ligands from Natural Peptides. R01-HL17044 (Valdivia HH, Van Petegem F, PIs).