Multidisciplinary team performs minimally-invasive transcatheter mitral valve repair
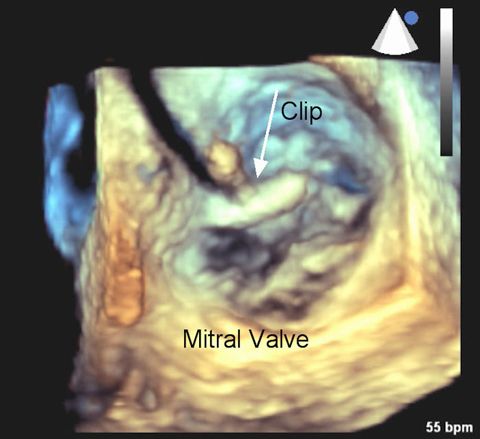
Transcatheter Mitral Valve Repair (TMVR) is indicated for patients with degenerative mitral valve regurgitation (MR) who are considered high risk for surgery. During MR, incomplete closure of the mitral valve causes blood to flow backward through the valve and into the left atrium. The 3-D echocardiogram at right shows the mitral valve leaflets from the perspective of looking downward on the valve. The clip is being put into position. (Image courtesy of Dr. Peter Rahko)
UW Health cardiac patients living with degenerative mitral valve regurgitation (MR) who are too sick for open-heart surgery now have a new option: minimally-invasive Transcatheter Mitral Valve Repair (TMVR) with a MitraClip device. On November 15, 2016, members of the multidisciplinary UW Health valve team successfully performed the first two TMVR procedures conducted at our institution.
“We are happy to be able to offer this option to some of our sickest patients, and are extremely grateful for the hard work and dedication of the faculty and staff who contributed and participated in this effort,” said Giorgio Gimelli, MD, associate professor (CHS), Cardiovascular Medicine and medical director of the UW Health Transcatheter Aortic Valve Replacement (TAVR) program.
Patients with degenerative MR experience backward flow of blood into the left atrium due to incomplete closure of the mitral valve. The condition is common, affecting 2 percent of the population. Approximately half of patients with severe MR are not treated using conventional open-heart surgery because of advanced age (nearly 10 percent of individuals aged 75 or older has moderate or severe MR), reduced left ventricular function, co-morbidities, or other contraindications to open-heart mitral valve surgery. One-year mortality for MR is 57 percent due to progression to heart failure and death.
TMVR is catheter-based therapy performed using venous access and real-time imaging (echocardiography and fluoroscopy) to place the MitraClip device in the center of the valve. The device grasps and coapts the mitral valve leaflets, resulting in fixed coaptation of the mitral leaflets throughout the cardiac cycle. The device gained FDA approval in 2013. More than 1,200 patients in the United States have been treated with this procedure in clinical studies, with 1-year follow-up in over 900 patients. Over 35,000 patients have been implanted with the device worldwide.
A review of comparative studies of MitraClip versus surgical repair for MR published in November, 2016 concluded that the procedure achieves similar survival to surgical mitral valve repair despite higher patient risk profiles, but recurrent MR occurs 4.8-fold more frequently after the MitraClip than surgical repair (Takagi et al).
For more information on TMVR with MitraClip at UW Health, contact the Heart Valve Clinic at University Hospital at (608) 263-1530.
Resources:
- Transcatheter Mitral Valve Repair With MitraClip Therapy (UW Health)
- Takagi H, Ando T, Umemoto T; ALICE (All-Literature Investigation of Cardiovascular Evidence) Group. 2016. A review of comparative studies of MitraClip versus surgical repair for mitral regurgitation. Int J Cardiol. 228:289-294.
- MitraClip (information for healthcare professionals)