Innate Immunity, Airway Remodeling, and Epigenetic Reprogramming
Dr. Allan Brasier is a physician-investigator internationally recognized for his scholarship on how cellular signaling pathways induce innate inflammation, a process associated with many chronic diseases. His work has advanced the understanding, at the cellular level, how innate signaling is activated and the intracellular signaling pathways involved.

Research Overview
Innate Immunity
Mucosal surfaces, such as the airways, are under constant attack by bacterial and viral pathogens. The first line of protection against these pathogens is mediated by a “nonspecific” immune response, termed innate immunity.
Innate immunity is an inflammatory response by the recognition of molecular patterns indicating the presence of replicating organisms. These molecular patterns are recognized by pattern recognition receptors on the surface of epithelial cells and others. Once triggered, pattern recognition receptors produce the expression of inflammatory and anti-viral genes by the epithelium. These proteins result in shaping the adaptive immune response important in specific immunity.
NFκB Activation
Dr. Brasier's research has demonstrated the central role of a transcription factor, termed NFκB, and the mechanism of how it activates inflammatory gene expression. NFκB activates a rapid, regulated gene expression mechanism termed “transcriptional elongation” to mediate innate response. Not only does this pathway provide an acute response to new viral infections, it controls cellular reprogramming, cell-state transition and remodeling/fibrosis. This is a fundamental discovery that has significant translational implications.
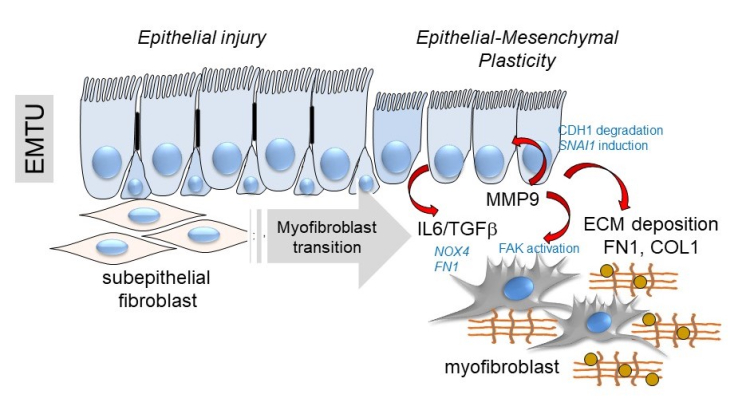
Below, a schematic of the transition of the epithelial-mesenchymal trophic unit (EMTU) of the small airway.
Research Team

There are opportunities for motivated undergraduates, graduate students and postdocs in the Brasier Lab! If you are interested in joining the group, please send your CV and a brief description of your research experience and interests directly to Dr. Brasier.
Active Projects
- Innate Signaling in Viral Induced Airway Disease
-
Our lab demonstrated the effect of RSV replication on NFkB activation in infected epithelial cells. In this setting, NFkB activation is controlled by changes in subcellular location and through site-specific phosphorylation. We have pioneered the findings that NFkB activation is closely intertwined with the DNA damage response.
Our more recent work has demonstrated that the DNA base excision repair protein, OGG1, complexes with NFkB to make an epigenetic complex controlling epithelial plasticity, and innate immunity. Current questions being examined include the role of the epithelium in controlling the innate inflammatory response using tissue specific inducible Cre-Lox systems.
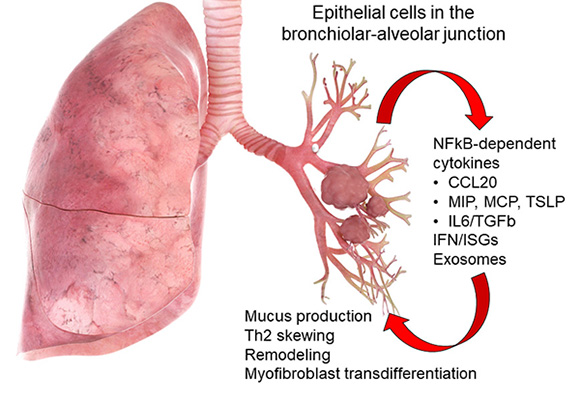
Above, role of the tracheobronchiolar epithelial cell in innate inflammation and remodeling. Schematic view of the lung with actions of the tracheobronchiolar cell in mediating viral innate responses. &Distal small airway cells preferentially elaborate cytokines associated with Th2 polarization and airway remodeling. These include CCL20, a mucin inducing cytokine, thymic stromal lymphopoietin (TSLP), a chemokine involved in Th2 polarization, and growth factors IL6 and transforming growth factor β (TGFβ).
- Systems Level Studies of the NFκB-BRD4 Pathway
-
We are approaching a deeper understanding of the NFkB network in airway epithelial cells using next generation technologies, such as RNA-Seq, chromatin immunoprecipitation (ChIP)- Seq, and protein interaction studies. Using affinity protein interactions, we have applied unbiased proteomic studies of NFkB, CDK9 and BRD4 interactomes.
Current questions being examined include the proteins interacting with BRD4 how this complex is affected by innate signaling..
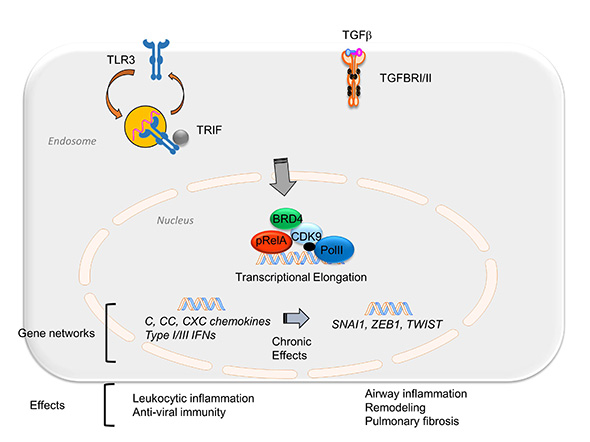
Above, integration of the NFκB-BRD4 in inflammation and fibrosis. TGFβ or poly IC stimulation activates an NFκB-dependent transcriptional elongation complex. Short term stimulation produces expression of the innate inflammatory response including chemokine and mucosal IFN production. Chronic or repetitive stimulation triggers expression of the core EMT regulators SNAI1, TWIST, and ZEB through transcriptional elongation process. In this way, the NFκB-dependent BRD4 recruitment is a major determinant of chronic airway inflammation, type II mesenchymal transition of airway epithelium, and pulmonary fibrosis.
- Mechanisms in Airway Remodeling
-
Repetitive allergen and viral exposures produce structural (fibrotic) changes in airway cells known as airway remodeling, associated with decreased lung function. Remodeling involves metabolic adaptations of the epithelial cell, producing expansion of the subepithelial myofibroblast population, and enhanced extracellular matrix production. We are investigating the coordination of the NFkB pathways and the unfolded protein response in mediating this response.
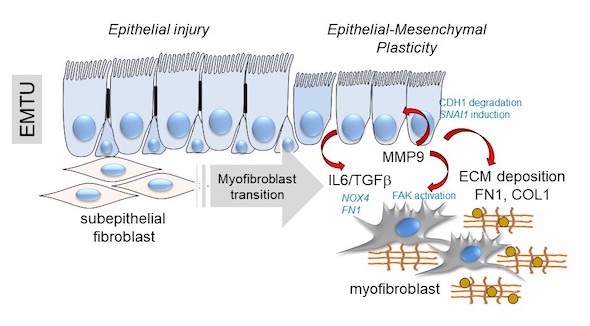
Above, a schematic of the transition of the epithelial-mesenchymal trophic unit (EMTU) of the small airway. Under resting conditions, the small airway epithelium interacts with a thin sheath of subepithelial fibroblasts. In response to epithelial injury produced by respiratory viruses or aero-allergens, growth factors and matrix metalloproteinases released by injured epithelium trigger the subepithelial fibroblasts to acquire pro-fibrotic characteristics including expression of aSMA, FN1 and COL1 resulting in ECM expansion of the lamina reticularis of the airway.
Funding Support
Dr. Brasier's research is funded by the National Institutes of Health (National Center for Advancing Translational Sciences and National Institute of Allergy and Infectious Diseases), and the Dr. Ralph and Marian Falk Medical Research Trust.